APC-400
(tempol) capsule
DMK Pharma has a worldwide license to use Tempol for the treatment of respiratory diseases including asthma, respiratory syncytial virus, influenza and COVID-19.
Potential Indication: Respiratory Disease (COVID-19, influenza, RSV)

In August 2021, Adamis announced (Stanford Study) the results of a published study in collaboration with Stanford University researchers suggesting that Tempol has strong, broad in-vitro anti-cytokine activity. Suppression of inflammatory cytokines with an antioxidant may be a beneficial treatment strategy in early COVID-19 infection.
Additional published studies, in which animals were infected with betacoronavirus, show that Tempol treatment resulted in increased survival and decreased viral load. Taken together, this scientific data argue for the use of Tempol in preventing and treating the most severe death related lung manifestation of COVID-19. Tempol has already been shown to be well tolerated in human clinical studies to date.
 |
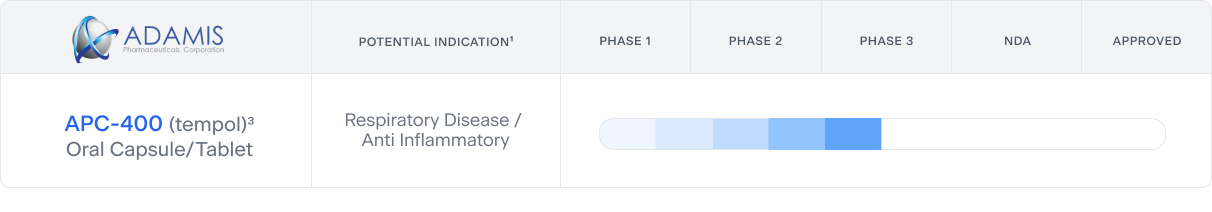
POTENTIAL INDICATION1 | PHASE 1 | PHASE 2 | PHASE 3 | NDA | APPROVED |
|---|---|---|---|---|---|---|
| APC-400 (tempol) Oral Capsule/Tablet | Respiratory Disease / Anti Inflammatory |
|
||||



